
生物分析(生命科学)
BIOANALYSIS
Service introduction
Small Molecule Bioanalysis
The business of chemical bioanalysis was officially launched in 2015. With the entity operation in China and the United States, the company can support the bioanalysis business in China and abroad with high efficiency and quality. The company's small molecule bioanalysis business has rich experience in areas of high technical difficulty, such as inhalants, liposomes, high sensitivity, instability, endogenous drugs, peptide drugs, etc. At present, more than 580 analytical methods have been established, including more than 330 chemical drug methods owned by the company. A total of nearly 800 consistency evaluations and nearly 150 clinical sample analyses of small molecule chemical innovative drugs have been completed.(Data up to 2022.06.30)
Xihua is also equipped with special QC, QA, sample management and equipment management personnel in accordance with a certain proportion to support the efficient operation of biological analysis of chemical drugs.

Nearly 580 bioanalytical methods for
chemical drugs, including more than
330 chemical drug methods with proprietary intellectual property rights

Experienced with difficult methods such as inhalers, liposomes, high sensitivity, instability, endogenous drugs, insulin, etc.

Roughly 600 domestic and international clients
Most of the top 50 domestic and the top 10 global pharmaceutical enterprises
also covers small and medium-sized Biotech enterprises

PK & BE statistics analysis
Statistical analysis of safety and efficacy for innovative drug

Clinical sample analysis:
1000/week/project/instrument

10家战略合作临床CRO
国内20多家战略合作临床基地
Large Molecule Bioanalysis
Large molecule bioanalysis business, established in 2015, has a complete quality system, systemically verified instruments and electronic systems, and can provide clinical sample bioanalysis services that meet the requirements of Chinese and Western drug application. Xihua large molecule bioanalysis Group is mainly engaged in the biological analysis of clinical samples of monoclonal antibody, polyclonal antibody, ADC, fusion protein, peptide, gene therapy, vaccine, CAR T, heparin, coagulation factor and other types of drugs. It can support the detection of PK, ADA, Nab (combination assay and cell assay), biomarkers, in vivo efficacy, flow cytometry, ELISPOT, etc. In addition, in accordance with a certain proportion, Xihua is also equipped with additional personnel dedicated to chemical QC, QA, sample management and equipment management, to support the efficient operation of macromolecular biological analysis.
The company has accumulated about 370 analysis methods, including more than 40 biological drug analysis methods with proprietary intellectual property rights, completed nearly 100 biological innovative drugs and biosimilar drug clinical trial sample analysis, completed biological analysis projects have more than 70 drugs approved for market. (Data up to 2022.06.30)
Xihua large molecule bioanalysis operates in both China and the United States, and can support the business in China and abroad with high efficiency and quality.
01
Development and validation of the analytical methods of PK, ADA, Nab and biomarker of macromolecules
02
Analytical methods developed for 40+ biosimilar drugs and nearly 100 innovative drugs
03
PK statistical analysis
Statistical analysis of efficacy and safety
04
Clinical sample analysis:
500 samples/week/project
05
Reagent development and labeling
Project Experience
Small Molecule Project Experience
| Regular chemical drugs |
Peptide |
| ADC drugs (toxin and linker) |
Oligonucleotide (AS0, siRNA) |
| PROTAC molecule | Biomarkers (including endogenous compound) |
| Metallic drug | Inhalant |
| Liposome | Metabolite identification of innovative drug |
Project Expexiences On Large Molecule Bioanalysis(Partial)
| BIOSIMILARS | OTHER | INNOVATIVE |
| Adalimumab | PEG - GCSF | Monoclonal Antibodies |
| Bevacizumab | FSH | ADC |
| Denosumab | Etanercept | Peptides |
| Ramucirumab | Insulin Degludec | PEGylated Drugs |
| Rituximab | Exenatide | Fusion Protein ( Fc , CTP ) |
| Panitumumab | liraglutide | Coagulation Factors |
| Pertuzumab | Semaglutide | Enzymes |
| Trastuzumab | Enoxaparin Sodium | Blood Products |
| Cetuximab | Nadroparin Calcium | Nucleic Acid Drug |
Large Molecule Platform
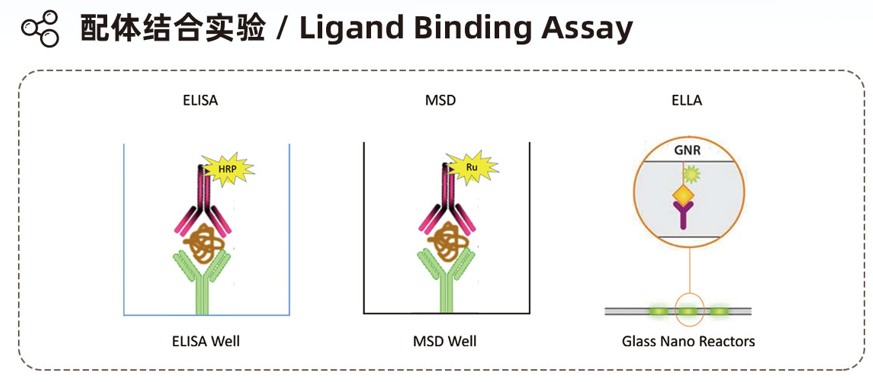
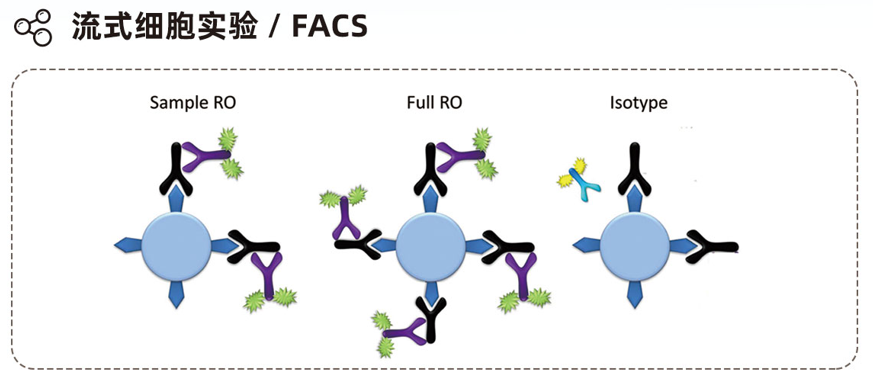


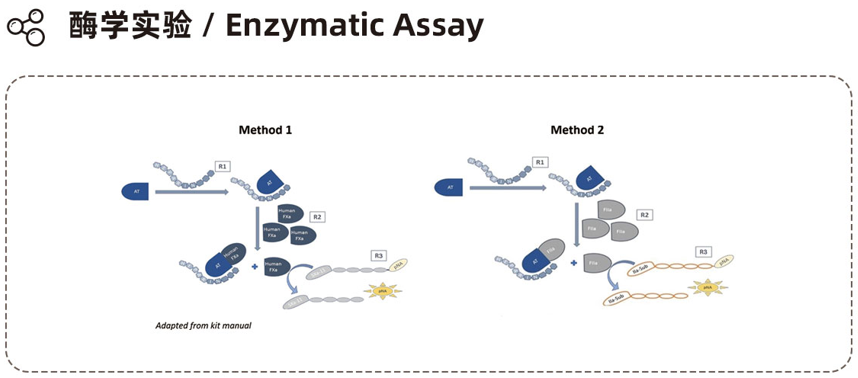




Experimental Instruments
Small Molecule Bioanalysis




-
LC/MS:Triple Quad 7500, API 6500plus,
API 6500,API 5000,API 4000 -
Agilent ICP-MS
-
Agilent GC-MS
-
Agilent FTIR
-
UPLC: Shimadzu/Waters
-
Automation: Cybio liquid workstations
-
LIMS: Watson LIMS
-
LC-HRMS-UV
Large Molecule Bioanalysis



- Electrochemical luminescence: MSD
- ELISA: MD Microplate Peader
- Flow cytometry: BD Fortessa X-20R
- q-PCR: AB QuantStudio 6/12k Flex System
- dd-PCR: Bio—Rad QC200
- Ultra-sensitive automatic microfluidic immunologicaldetection platform:
ProteinSimple Ella Simple Elisa - Single molecule immunodetector: SET SMCxPRO™, SIMOA
- Bio-plex 200 suspension chip system: unit
- CTL ELISA spot analyzer: unit
- Hba1c analyzer : Tosch G8
- Coagulometer system:Sysmex CS-2400+CA-620
- Automation: Tomtec/Hamilton/Tecan liquid handlers
- Plate washer: Bio-Tek
- Watson LIMS (including Immune model)
Quality system
Service advantages

全内资
无需通过遗传办

稳固的团队经验与技术
项目负责人经验
本科至少8年
硕士至少5年

与NMPA合作
持续开展生物分析行业培训提供咨询服务

质量至上
超过110次中美核查

严守时限
创新药爬坡
小分子:48小时提供结果
大分子:72小时提供结果

First-mover advantage
Deeply engaged in bioanalysis and
rich experience in the field of bioanalysis

Solid Team Experience and skills
Project leaders have many years of industry experience

International Service
Able to meet customers' needs for China-US dual declaration and global multi-center clinical declaration

Quality
As of 2022.06.30
More than 110 inspections by NMPA and FDA

Punctuality
Dose escalation of
innovative drug

Xihua Headquarters
Address:Building 5, 6 and 7, Lane 118, Furonghua Road, Pudong New Area, Shanghai
Learn more>Business Consulting
Overseas
Tel:+01-609-921-7115 (US)
Fax: +01-609-921-7116 (US)
Other Consultations
China
Email:IR@xihuasci.com








